Yeah, so I started a new clinical trial almost two weeks ago. This one, like the others, is being conducted out of the Center for Diabetes Technology at the University of Virginia, and also at UC Santa Barbara and the Mayo Clinic in Minnesota.
If you missed any of my other references, I’ll tell you straight out: This trial has an artificial pancreas element to it. That’s exactly how I’m describing it, because really, the trial is literally weeks worth of work by myself and others to prepare for about 36 hours on a closed loop artificial pancreas system. Here’s the sequence of events as I know it right now:
First, I went to Charlottesville to get screened for the study. This meant going through a physical, getting blood drawn for an A1c, having an EKG performed, the whole nine yards. We also went through the consent form (about 20 pages or so), and I submitted my medical history. This included things like confirming I was Type 1, that I’m not currently seeking treatment for things like alcohol or drug addiction, listing any hospital stays I’ve ever had, and listing out all of the medications I take on a daily basis, and in what amounts. The hardest part about these visits is that my BGs get so ramped up driving down to Charlottesville that I’m always running high when I arrive. The Jersey Turnpike has nothing on Interstate 81.
After the screening visit, I drove back home (more stress—I don’t know if I made it below 200 mg/dL all day) and waited for the phone to ring, telling me whether I had been accepted or rejected from the study. I had been rejected from two previous AP trials (different reasons), so I wasn’t holding my breath over this one. But I was accepted, and I was thrilled.
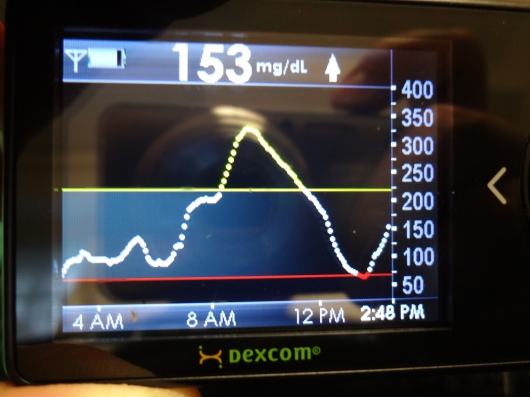
The following week I started a week of data collection by inserting a Dexcom sensor. I also started using a meter given to me to specifically be used for this trial. And I started keeping a daily diary. It included information on whether I was sick, whether I did a pump site or CGM sensor change, how I was feeling, how stressed I was about hyperglycemia and hypoglycemia, and whether I worked out that day. In addition, I needed to record times, BG fingerstick numbers, and carb counts of everything I put into my mouth. For an entire week. At the end of the week, I uploaded pump, CGM, and meter data, and faxed the daily diary info to the research team.
What was the purpose of all this? So the team at UVA could use my data to help build an algorithm specifically for me. When we finally get to the point where I’m hooked up to the closed loop system, the algorithm will work to help anticipate BG trends and carb intake, and make updates to insulin delivery as a result. Cool, yes?
So what’s next? Next are two separate admissions in the Center for Diabetes Technology’s research house in Charlottesville. I don’t know which visit will be which yet, but I do know that one of the visits will involve being on the closed loop artificial pancreas system itself, and one visit will not. The idea, as I understand it, is for the team to compare results on the closed loop system versus results from an open loop system in which I’m making decisions on my own, just like I do today.
My first admission is next week. Wish me luck! Actually, no… wish the dedicated team of researchers, developers, doctors and nurses luck. They’re doing the hard work of creating something that could be a game changer in terms of insulin therapy for people living with diabetes. Remember this: In all of these studies conducted, there have been zero overnight lows. My hope is that I can help further research into this and other advancements that are making the lives of the newly diagnosed better than I ever could have imagined at my diagnosis back in 1991. You and I are worth the effort.
I’ll try to give as much of a play-by-play account of what’s going on as I progress through the trial. Watch this space and Twitter for more.